
Calcium channel blockers are important therapeutics for a broad range of cardiovascular and neurological diseases including hypertension, Parkinson’s disease, and chronic neuropathic pain. Presently, there are several limitations to calcium channel blocker therapy including serious off-target effects and lack of small molecules that distinguish effectively among the different calcium channel types. To address these shortcomings, the Colecraft lab has been studying a family of four proteins Rem, Rad, Gem, and Rem2—that powerfully inhibit all calcium channels. They have discovered that this seemingly homogenous and simple phenomenon is underlain by a rich variety of mechanisms and binding sites. The insights from this work offer clues for the design and development of novel, selective genetically-encoded and small-molecule calcium channel blockers for potential therapeutic applications. They are currently pursuing this aim.
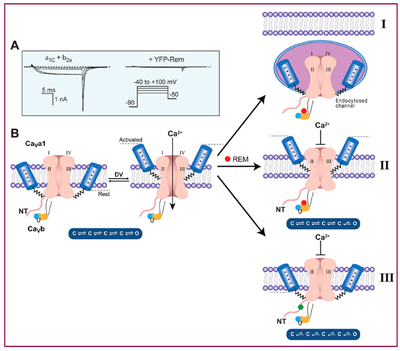
Rem inhibits CaV1.2 channels using multiple mechanisms and binding sites.
(A) Left, Calcium currents reconstituted in HEK 293 cells. Right, currents are inhibited in the presence of Rem. (B) Rem inhibits calcium current using three independent mechanisms: by removing channels from the cell surface (mechanism I); by reducing the open probability of surface channels (mechanism II); by restricting the movement of channel voltage sensors (mechanism III). Mechanisms I and II do not require GTP to be bound to Rem (red hexagon) whereas mechanism III does (green hexagon).
page 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |> NEXT
> download 2013 newsletter PDF
> download 2009 newsletter PDF
> view 2007 newsletter

